Multiple Sclerosis
In Vivo Models for Identifying Key Endpoints and Testing Novel Therapeutics

Multiple sclerosis is a chronic neurological disorder where auto-reactive T cells attack the myelin sheath, disrupting nerve communication and causing symptoms including fatigue, muscle stiffness, paralysis, sensory loss, and cognitive impairment. Despite treatment advances, there is no cure for multiple sclerosis. Ongoing drug development is vital to discover more effective therapies, reduce disability, and improve patient outcomes, ultimately aiming for a cure. Inotiv offers a relevant preclinical in vivo model of multiple sclerosis and related research services for developing novel multiple sclerosis therapeutics.
EAE Model of Multiple Sclerosis
Experimental autoimmune encephalomyelitis (EAE) is a demyelinating central nervous system (CNS) disease commonly used as a model for multiple sclerosis due to its shared clinical and pathological features. EAE is induced in animal models through immunization with myelin proteins, such as myelin-oligodendrocyte glycoprotein (MOG), accompanied by pertussis toxin (PTX), which activate myelin-specific T cells that migrate to the CNS, triggering inflammation, demyelination, and nerve injury. EAE models allow for the preclinical testing of a wide range of potential therapeutic interventions and provide valuable insights into the immunopathogenic mechanisms of multiple sclerosis.
Study Design

Validation data

EAE was induced in C57BL/6 mice by immunization with the MOG(35-55) peptide. EAE mouse models were treated (after enrollment) with fingolimod (5 mg/kg, PO; grey line), prednisolone (10 mg/kg, PO; pink line) or a vehicle control (orange line). Clinical scores of treated disease animals, untreated non-disease mice (black line), and untreated disease mice (red line) were recorded. Data sets that do not share any letters differed significantly.
Histopathological Services for EAE Models
Histopathological assessment of spinal cords and brains from EAE models provides detailed insights into the disease's pathological mechanism. Brain and spinal cord sections are stained with hematoxylin and eosin (H&E) or luxol fast blue (LFB) and examined microscopically by a board-certified veterinary pathologist to determine inflammatory infiltrate and demyelination, respectively, which are key features of multiple sclerosis. Histopathological analysis of neural tissue from EAE models helps in understanding how potential treatments affect these pathological processes, enabling the identification of effective therapies that can reduce or reverse the damage.
Learn more about our range of specialty histology and image analysis services.
Validation Data
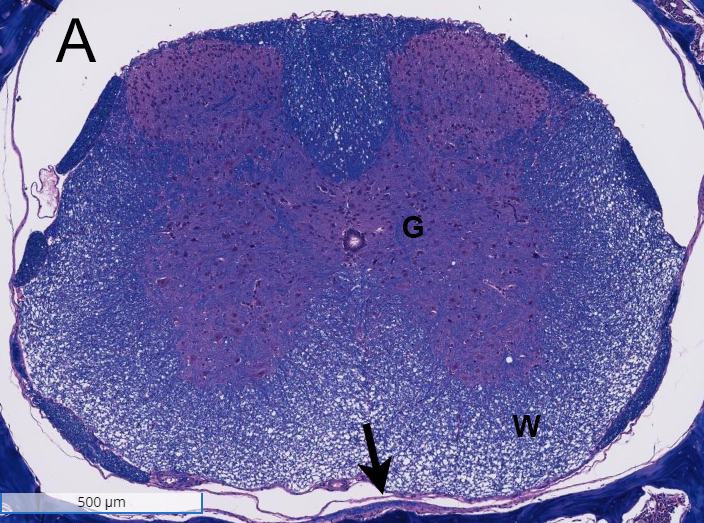
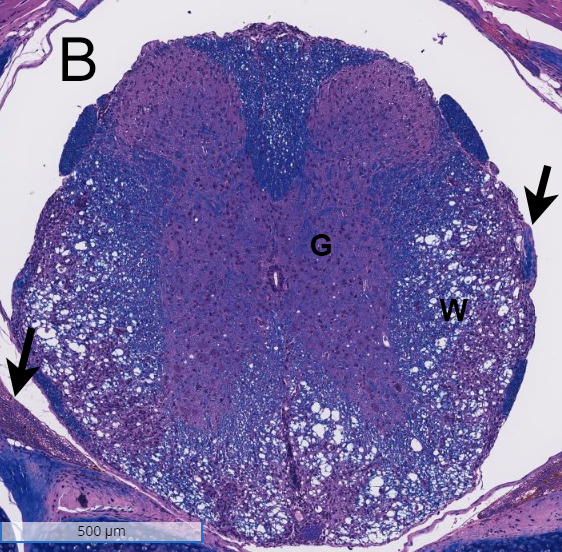
Spinal cords from a non-disease animal (A) and a EAE mouse model (B) were stained with H&E and analyzed for inflammation and demyelination. Inflammation was observed in the white matter (W) of the spinal cord from the EAE mouse model, and demyelination was noted in the meninges (arrows). G= grey matter.
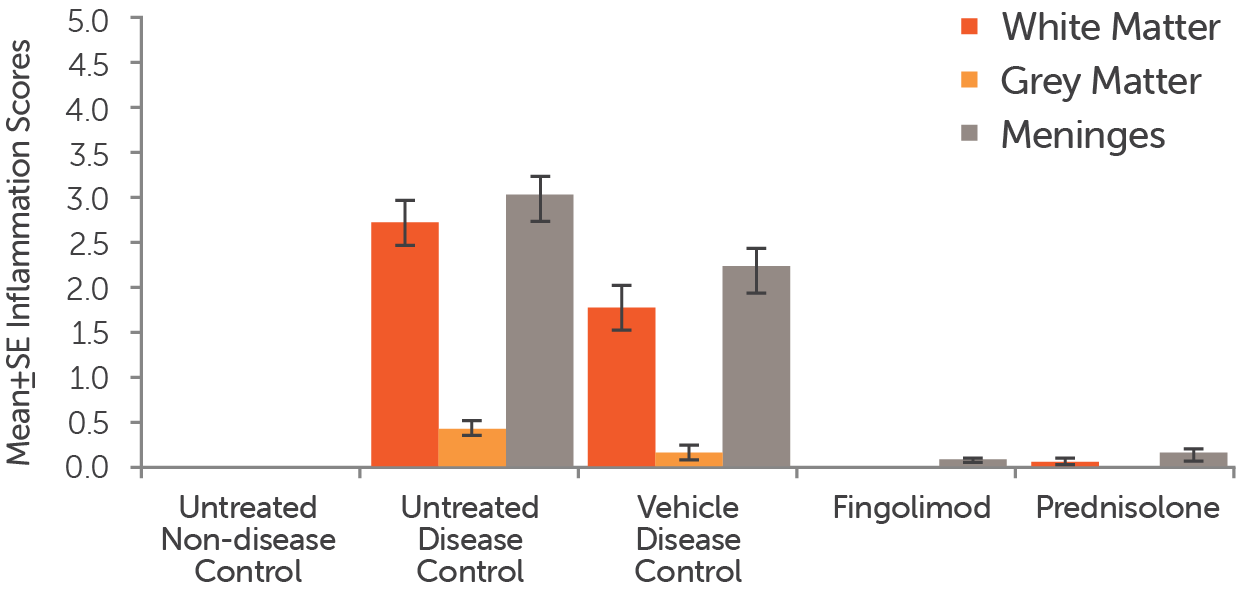
Stained sections of spinal cords from EAE mouse models that were treated (after enrollment) with fingolimod (5 mg/kg, PO), prednisolone (10 mg/kg, PO), or a vehicle control, as well as untreated non-disease and untreated disease mice, were analyzed for inflammation. Inflammation scores for the white matter (red bars), grey matter (orange bars) and meninges (grey bars) in the animals were recorded.
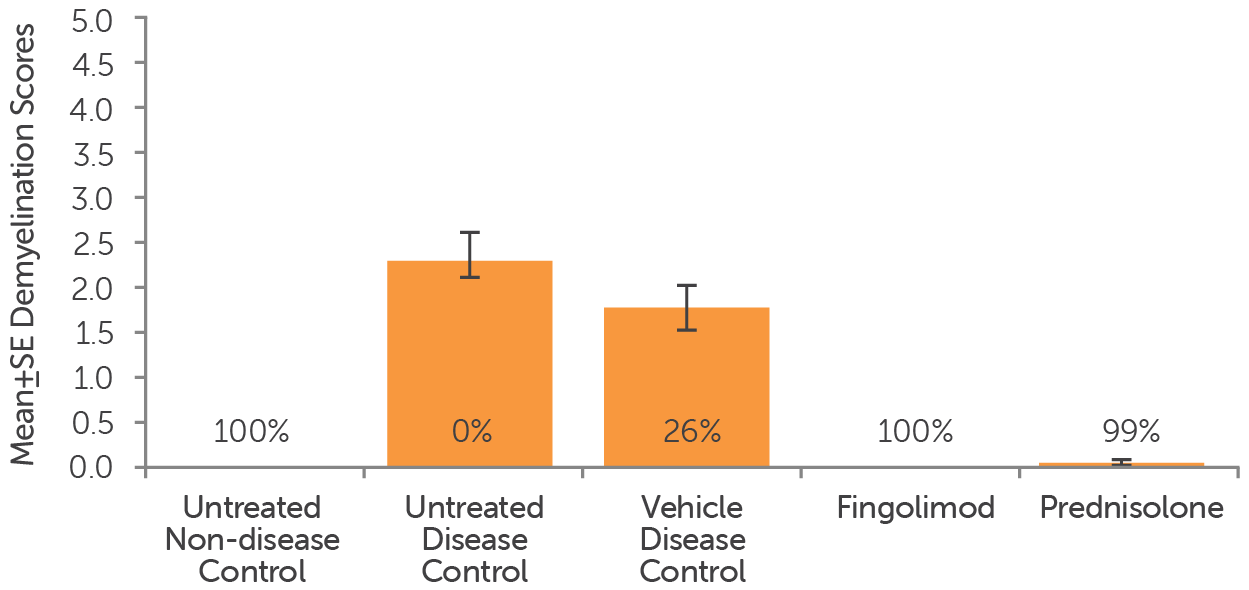
Stained sections of spinal cords from EAE mouse models that were treated (after enrollment) with fingolimod (5 mg/kg, PO), prednisolone (10 mg/kg, PO), or a vehicle control, as well as untreated non-disease and untreated disease mice, were analyzed for demyelination. Demyelination scores for the animals were recorded.
Additional Endpoints
Our capabilities extend beyond models and behavioral testing. We have a broad range of GLP and non-GLP in vivo and in vitro assays that can be customized to provide solutions for your multiple sclerosis drug discovery program.
- Stereotaxic surgery
- Tissue harvesting
- Oxidative stress enzymology
- Neurotransmitter and metabolite quantification
- CBC/clinical chemistry analysis
- Mass spectrometry proteomics
- Primary neural cell culturing
- Human stem cell and brain organoid culturing
If you do not see a capability you need, please contact us as we are regularly developing new models and processes to meet our clients’ needs.